X射线荧光光谱仪样品制备方法:压片法介绍和注意事项
样品制备技术对于在分析样品时获得更好的结果至关重要。样品制备技术会影响X射线荧光分析结果的准确性。
样品(简称标样)的配制
X射线荧光分析与化学分析不同,荧光分析仪是一种相对测量仪器,它是通过测量一定数量已知结果的标准样品,建立相应的正确的数学模型后,才能得到准确的测量。也就是说:要达到好的测量效果,一组好标样与一台好仪器同样重要。好仪器由我公司提供,好标样得由用户化验室提供。好标样的标准是:有代表性,有一定的跨度范围,有准确的化学分析结果。具体要求如下:
●样品量:5-10.每个质量:50-100克
●具有代表性:样品可以代表您工厂的实际产品,是来自实际生产线的瞬时样品,尽量避免人为准备的样品,这会带来主观因素。并且不使用国标样品,每个工厂使用不同的原材料和不同的配方,会得到不同的数学模型。
●主要化学成分的含量范围。
压片制备步骤
标准样品中主要化学成分的含量范围应涵盖正常产品主要化学成分含量的变化范围,且应保持距离,分布均匀。以水泥原料为例:正常情况下,在原料(白色原料)中,如果CaO含量目标值为42%,那么,为了保持距离,CaO含量的最小值约为39 %,值高为45%左右,为使CaO含量分布均匀,CaO含量应为39%、40%、41%、42%、43%、44% ,45%。您可以额外准备几个此类标准样品,其含量在正常变化范围内(41%~43%)。 (表 1)
是一系列白色原料的标准样品,主要化学成分的目标值分别如下:
Al2O3:3.1%,SiO2:13%,CaO:41.5%,Fe2O3:3.5%
在表1中,我们可以得到:主要原料的含量之间保持一定的距离,分布均匀。 Al的变化范围为2.5%,Si的变化范围为4-6%,Ca的变化范围为4%-6%,Fe的变化范围为3%。有铝与硅、硅与钙、钙与铁之间没有人为的规律性。
一般来说,高含量元素的变化幅度大于低含量元素的变化幅度,含量越低,变化幅度越小
表1.白生料标样结果
Na2O | MgO | Al2O3 | SiO2 | SO3 | K2O | CaO | Fe2O3 | |
1# | 0.22 | 2.84 | 3.46 | 16.38 | 0.66 | 1.08 | 38.60 | 3.46 |
2# | 0.38 | 2.50 | 3.73 | 15.79 | 0.18 | 1.31 | 38.89 | 3.24 |
3# | 0.22 | 1.97 | 3.57 | 15.61 | 0.18 | 1.01 | 40.03 | 3.59 |
4# | 0.18 | 1.79 | 3.06 | 13.92 | 0.16 | 0.94 | 40.42 | 5.29 |
5# | 0.43 | 1.93 | 3.48 | 14.72 | 0.45 | 1.17 | 40.39 | 3.44 |
6# | 0.17 | 3.28 | 2.87 | 12.16 | 1.18 | 0.92 | 40.66 | 3.29 |
7# | 0.16 | 2.25 | 4.38 | 12.93 | 0.30 | 0.96 | 40.92 | 3.50 |
8# | 0.17 | 1.92 | 3.19 | 13.62 | 0.18 | 0.98 | 41.22 | 4.33 |
9# | 0.18 | 1.81 | 3.04 | 13.04 | 0.16 | 1.10 | 41.72 | 3.54 |
10# | 0.56 | 1.82 | 4.10 | 11.96 | 0.75 | 0.87 | 41.94 | 3.21 |
11# | 0.26 | 2.30 | 2.80 | 11.37 | 0.11 | 1.11 | 43.03 | 2.11 |
12# | 0.12 | 1.94 | 2.53 | 10.50 | 0.15 | 0.75 | 44.30 | 2.66 |
13# | 0.11 | 1.70 | 2.09 | 8.84 | 0.14 | 0.70 | 45.86 | 2.26 |
一般,系列标样中对于含量为40%、50%、60%、70%、80%左右的元素(或其氧化物),其跨度范围应为:5%-10%左右;含量为10%、20%、30%左右的元素,其跨度范围应为:4%-8%左右;含量为1%-10%左右的元素,其跨度范围应为:3%-5%左右。即:含量较高的元素,跨度范围应相应增大;含量较小的元素,跨度范围应相应减小。
l 系列标样里高低样的制备:
系列标样中,对于目标值附近的样品,直接从生产线上留取;而对于系列标样中的高低样品,一般很难从正常生产中的生产线上取出,这种情况下,我们只好人为配制高低样品。仍以表1中的白生料为例,正常生产时白生料CaO的含量一般控制在41.0%-42.0%左右,要达到45%的高CaO含量,我们在留取的正常生产样中,按一定比例添加少量石灰石原料,以使CaO含量升高、同时使Al2O3、SiO2、Fe2O3含量降低,可达到高CaO、低SiO2、低Fe2O3的目的。同理,可制备出高SiO3、高Fe2O3标样。CaO含量在正常范围内(40.0%-43.0%左右)的样品必须从生产线上留取,不可人为配制!
注意:应尽量减少人为配制标样。当需要人为配制标样时,应采用在大量的实际生产样中添加少量原材料的方法,而且所添加的原材料必须是实际生产中所使用的同种原料。不要用同一结果的实际生产样去配制若干个标样(一个生产样最多只能配制一个人工标样),以减少人为的规律性。
l 混料:
当人为配制标样后,必须充分混合均匀。我们发现:许多厂家在混料方面都做得不够好。在样品本身不均匀的情况下就进行化学全分析和仪器测量,即使是同一种样品,化学分析时所取的样与仪器测量时所取的样很可能不一致,这样必将导致仪器测量值与化学分析值之间存在很大的误差,直接影响仪器测量的准确性。混料是一个比较枯燥但又十分关键的工作,混料是否均匀对后面的工作至关重要。如果这一环节做得不好,那么下面的工作即使做得再严格、再细致,也无济于事。混料要在*封闭的环境下进行,以减少轻基体元素含量的损失,避免造成标样与待测生产样品不一致。一般人工混合一个样品至少要15-30分钟。
l 化学分析:
荧光分析仪是以化学分析为基础的,如果化学分析本身不很准确,将来做出的数学模型误差就会很大甚至不能用。所以,为使化学分析结果准确,应由至少两名优秀分析人员各自做全分析,其分析结果误差在GB/T176-1996允许误差范围内时取其平均值作为最终结果。超差时应进行复验、删除错误值。分析过程中必须避免两人互相对比、修改分析结果。
l 保存:系列标样配制好后,用磨口玻璃瓶密封保存,贴上标签,注明编号、日期,以备长期使用。
二. 标准样品及待测样品的成型
用X射线荧光仪精确分析矿物样品需要适当的样品制备:所有样品经粗磨后还必须进一步细磨,在30MPa 压力下加压成型,以减小颗粒效应、矿物效应、元素间吸收-增强效应。
l 细磨
白生料:称量30g样品(精确到0.05g),0.2g硬脂酸(精确到0.0001g)(助磨剂,以防止细磨过程中结块,提高研磨效率),放入振动磨中,细磨2分钟。
熟料:称量30g样品(精确到0.05g),放入振动磨中,往磨中加10滴乙醇,细磨2分钟。
石膏、铁粉:称量30g样品(精确到0.05g),1g硬脂酸(精确到0.0001g),放入振动磨中,细磨3分钟。
砂岩、页岩、粉煤灰:称量20g样品(精确到0.05g),2g硬脂酸(精确到0.0001g),放入振动磨中,细磨1分钟。
上述细磨时间仅供参考。用户应该根据本厂振动磨的研磨效率,通过实验来确定研磨时间。一般可用一参考样品,不断增加对它的研磨时间,同时测量其特征X射线强度,直至测得的强度不再升高(或降低)趋于恒值为止。注意:在细磨每个样品前,必须将振动磨的料钵清洗干净。细磨后的样品,放入小磨口瓶中保存,贴上标签,注明样品编号、日期。
助磨剂系分散剂,细磨时可防止结块,提高研磨效率,降低电耗。助磨剂种类较多,如硬脂酸、甘油、乙醇、丙醇、异丙醇、三乙醇胺、尿素等。标样和待测样必须使用同一种助磨剂,更换助磨剂种类时应重新制备标样细粉,重新压片后制作工作区。
l 压片
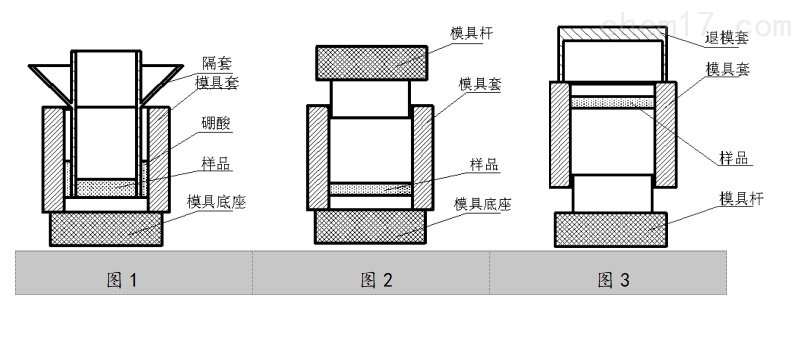
1. 磨具底座的上表面与模具套、隔套的内外表面擦拭干净,确保上面没有残留的样品和硼酸,并按(图1)组装好模具。
2. 称量7g(精确到0.05g)研磨均匀的样品,倒入隔套的中间,并用整平杆把样品摊均匀。
3. 称量7g(精确到0.05g)的硼酸,并将其中的1/2均匀地倒入模具套与隔套的中间;轻轻地取出隔套,并将剩余的硼酸倒在样品的上面,用整平杆将其摊平。
4. 按(图2)将模具杆装上。
5. 托着模具底座将整套模具放在压力机工作台的中间,将压力加到30MPa并停留30s后,把模具卸下。
6. 取下模具底座,按(图3)将模具倒置,并在上面放上退模套;手扶着退模套的中部,同时用胶皮榔头猛击退模套就可把样品退出(也可将模具放在压片机工作台的中间同时旋下压片机丝杠即可)。
7. 样品表面不得有沾污、擦痕、裂纹,否则重新压制,压片背面用彩色软笔注明编号、日期,然后正面朝上放入干燥器内。
8. 擦净模具等用具。模具长期不用时,应将其表面涂上一层黄油,以防止模具生锈。取放模具时要平稳,以免划伤底座,使压片表面不平。
The technique of sample preparation is essential for obtaining a better result when analyzing samples. the technique of sample preparation will affect the accuracy of the result of the X-ray fluorescence analyzing.
1.the preparation of standard samples
the X-ray fluorescence analyzing method is different from the chemical method, it is a comparative measuring method ,which gets the accurate result by establishing the corresponding mathematical model after measuring a certain mount known samples(standard samples) .in another word, a series of good standard samples is as important as a good instrument. a good instrument is provided by us, while a good series of standard samples is provided by the users’ laboratory. A good series of standard samples be of the following features: Be representative, Be of certain content range, and Be of accurate chemical analyzing result, the details are as follows:
●Sample amount:5-10.each mass:50-100gram
●Be representative: the samples can represent the actual products of your factory, and is the instantaneous samples from the actual production line, and try to avoid the artificial prepared samples, which will bring in subjective factors. and do not use national standard samples, for each factory use different raw materials and different formula, and will get different mathematical model.
●The content range of main chemical constituents.
The content range of main chemical constituents in standard samples should cover the variation range of content of main chemical constituents in normal products, and should keep distance with each other and be well-distributed. take the cement raw material as an example: in normal condition, in raw material (white raw material) if the target value of content of CaO is 42%, then, to keep distance, the minimum of the content of CaO will be about 39%,and the maximum of it will be about 45%,to make the content of CaO well-distributed, the content of CaO should be 39%(minimum),40%,,41%,42%,43%,44%,45%(the maximum). You may prepare extra several this kind of standard samples of which the content be in the normal variation range(41%~43%). (Tab 1)
It’s a series of standard sample of white raw material, and the target value of main chemical constituents is as following respectively:
Al2O3:3.1%, SiO2:13%, CaO:41.5%, Fe2O3: 3.5%
In tab.1, we can get that: the content of main raw material keep a certain distance from each other and be well-distributed. the variation range of Al(the maximum subtract the minimum) is2.5%, the variation range of Si is 4-6%, the variation range of Ca is 4%-6%, the variation range of Fe is 3%.there is no factitious regularity between Al and Si, Si and Ca, Ca and Fe.
Generally speaking, the variation range of high content element is larger than the variation of low content element, and the lower the content is , the smaller the variation range
●Preparation of the high content samples and low content samples.
In the series of samples, the samples whose content is near the target value can be collected from the production line. Those samples whose content is much higher or lower than the target is hardly available directly from the normal production line. We must prepare those sample by ourselves. Take the white raw material as example (Tab.1). In normal conditions, the content of CaO is controlled in 41%~42%,If we want to get the samples above 45%, we must add limestones in the raw material to increase the content of CaO and decrease the content of Al2O3, SiO2, Fe2O3, in the same way, we can prepare high SiO2 samples and high Fe2O3 samples. the samples whose content is near the target value must be collected directly from the production line.
NOTICE: try to avoid preparing artificial samples. When sample has to be prepared manually, you should add only a little raw material to a mass of practical
manufacture samples, and the raw material must be same as the manufacture samples. In order to reduce factitious regularity, users must not use the same manufacture material to prepare different samples(one manufacture sample can be use to prepare for only one manual sample).
●mixing material:
When users prepare sample manually, it must be well-distributed. We discovered that many users don't do well in mixing material .If the materials are not well distributed, even for the same sample, the parts for total-chemical analysis may be different from the parts for instrumental analysis, this may lead to large error between the chemical analytical values and instrumental analytical values, and then influence the accuracy of the instrument. Mixing material is critical for subsequent process though dreary, however, If this step is not done well, even if subsequent processes is done so strictly, so meticulously, the result will be beyond remedy. Mixing material must be done in a entirely sealed environment, for lessening the loss of light matrix element content. and avoiding the variance between sample and the things which will be tested. Generally, it needs 15-30 minutes to mix a sample manually.
●Chemical analysis:
fluorescence spectrometer analysis is based on chemical analysis, if chemical analysis is not accurate, the mathematical model will have great errors, even can not be used. So, for the sake of accuracy of chemical analysis, this step must be done by two excellent operator, and adopt the average as the final result when the error of each result is within the allowable range of the GB/T176-1996. if the error is oversized, users should check and delete and wrong value. In this process, the operaters must not compare with each other, nor juggle the results.
● storage:
when sample series is prepared well, seal them in ground glass bottles,and lable them for long-term use.
2:molding of standard samples and unknown samples
Mineral samples for XRF require proper sample preparation, all the samples need both coarse grinding and subsequent fine grinding, and molded under 30Mpa pressure to correct the granular effect, grain effect and matrix effect.
●Fine grinding
Raw material: Weight 30g samples(accurate to 0.05g),0.2g stearic acid(accurate to
0.0001g)(grinding aids, preventing caking and improving grinding efficiency), fill in vibration mill, grind for 2 minutes.
Clinkers: weight 30g clinkers(accurate to 0.05g),1g stearic acid(accurate to 0.0001g),fill in vibration mill, grind 3 minutes.
Gypsum, iron powder: weight 30g sample(accurate to 0.05g),1g stearic acid(accurate to 0.0001g),fill in vibration mill, grind 3 minutes.
Sand stone , shale, fly-ash: weight 20g sample(accurate to 0.05g),1g stearic acid(accurate to 0.0001g),fill in vibration mill, grind 1 minutes.
All the above grinding time is for reference only. Users should fix the best grinding time according to actual grinding efficiency. user can increase the grinding time of a reference sample, and at the same time, measure the intensity of the characteristic X-ray, until the right time that the intensity do not rise with the increase or decrease of grinding time.
NOTES: users must have the container of the vibration mill cleaned before grinding every sample. and the-grinded sample must be stored in ground bottle flasks, and labled with sample number, and date.
Grinding aid plays the role as dispersant, preventing caking, improving grinding efficiency and reduce energy consumption. there are many sorts of grinding aid, such as stearic acid, glycerol and alcohol, propanol, isopropanol,
triethanolamine,and urea etc. Standard samples and unknown samples must use the same grinding aid. changing the grinding aid must change the standard sample powder, and squash again and establish new work area.
●Squashing
1. The upper surface of the base of mill, the inner and outer surface of mill sleeve, must be cleaned and make sure that no residues and boric acid left. Assemble the mill according to Fig 1.
2. Weight 7g(accurate to 0.05g) grinded sample, and fill in the sleeve gasket and level the sample by leveling rod.
3. weight 7g(accurate to 0.05g)boric acid, fill half of it into the middle of the mill sleeve and sleeve gasket. drop out the sleeve gasket carefully, and fill in the left boric acid and level it.
4. Assemble the mill rod.(Fig 2)
5. Carry the mill to the center of the working platform of the presser, set the pressure to 30MPa, keep for 30s and disassemble the mill.
6. take the mill base out and invert the mill(Fig.3). Put on removal, and buttress the middle of the removal, then beat on the top of the removal with rubber hammer. you can take out the sample.
7. The sample should not be contaminated, scraped, or splitted, otherwise, you need to do it again. It is suggested to write the number, date on the back of the sample with the pastel.
8. Clean the mill. if the mill was left unused for a long time, grease should be smeared on the surface to prevent rusting. The mill should be carried carefully to avoid base scrape.